A Future Without Lab Animals
By Alexandra | December 11, 2023
Written by Alice Pettitt & Jun Jie Ong, Life Science Investors @ Creator Fund
Animal testing has long been the standard to assess the safety and efficacy of drugs intended for human use. In the UK, this involves testing all new drugs on rodents (usually mice or rats) and non-rodent mammals (usually dogs, pigs or monkeys).
Throughout history, animals have been repeatedly used for biomedical research.
The first known use of animals in scientific experimentation dates back to 500 B.C. in ancient Greece. For centuries the concept of animal cruelty was absent from the prevailing philosophies of the time, but since the 17th century, debates surrounding the ethics of animal testing have been ongoing and contentious.
Pharmaceutical companies and biotechs require tens of thousands of animals for testing each year. However over 95% of candidate drugs tested on animals in preclinical trials fail to demonstrate either efficacy or safety when tested in humans.
Now, perspectives are shifting globally and momentum is building for alternatives to animal testing. This change is being driven by increasing pressure from animal rights groups calling out the significant ethical concerns of testing drugs on animals and the use of animals failing to replicate human responses.
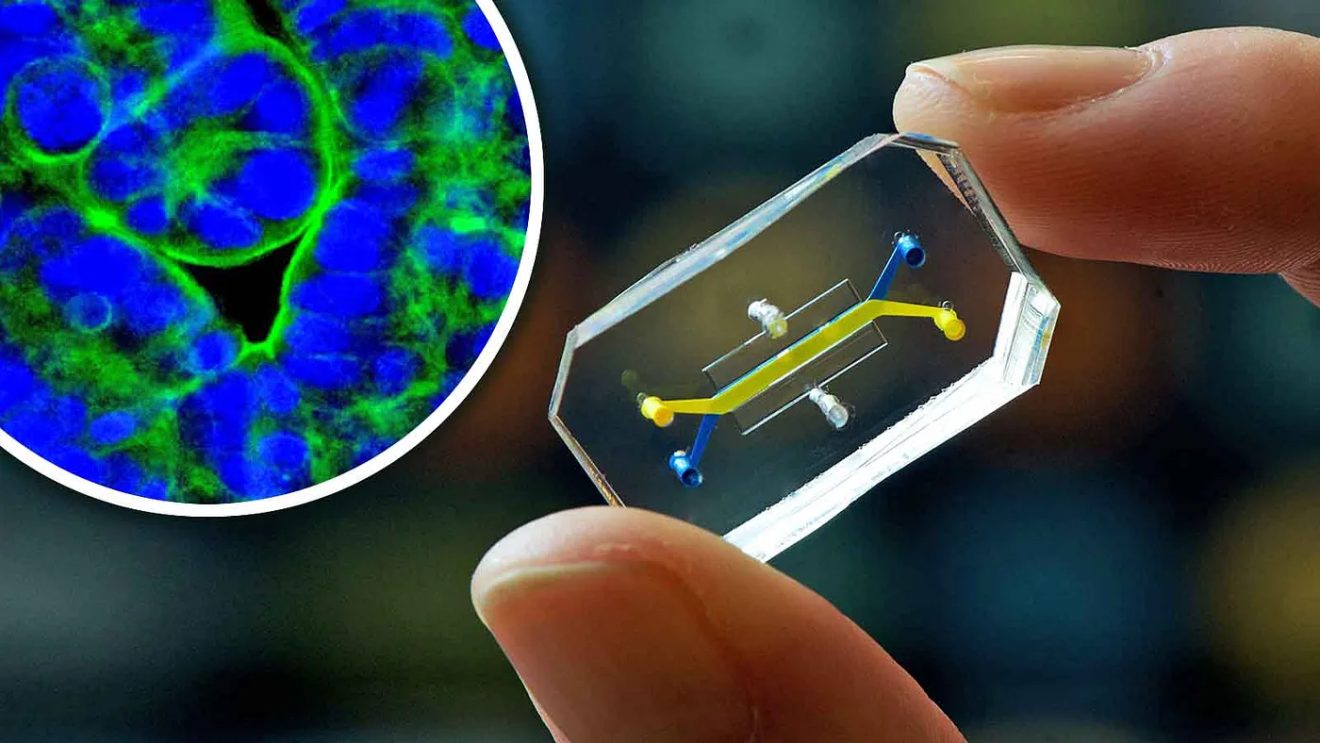
The need for alternatives
The primary objective of preclinical drug development is to gather sufficient data on a drug’s efficacy and safety, allowing for the determination of a safe starting dose for phase I in-human studies. The data collected during this stage is used to support the design and conduct of clinical trials.
While humans share many of the same genes as animals, the functions of these genes can diverge between species. As a result, many drugs that are tested in animals don’t work as well in humans. Conversely, differences between animal and human physiology also mean that substances deemed toxic to animals might be safe in humans, potentially causing many drugs to remain unexplored for human benefit.
Recent legislation will significantly accelerate the existing market trends for alternatives. Most notably, in 2022, the U.S. Food and Drug Administration (FDA) introduced the Modernization Act 2.0, which allows drug developers to collect initial safety and efficacy data using new non-animal methodologies. This new legislation allows the FDA to clear a drug for human trials without requiring animal testing and opens the way for the FDA and the pharmaceutical industry to discuss the suitability of alternatives.
In 2021 the European Parliament called for a plan to phase out animal testing.
Besides the current legislative tailwinds, the search for alternatives is also being accelerated by the development of innovative personalised therapies for patients, where many of these treatments may not show efficacy in animal models at all.

In vitro models
Over the past two decades, a rapid surge of novel technologies has revolutionised preclinical drug development. These technologies are model representations of living organisms and structures in an artificial environment (in vitro models) and are notably exemplified by organoids and organ-on-a-chip devices.
Organoids
Organoids are three-dimensional miniature models of organs grown in the laboratory. They are derived from stem cells or tissue samples and can mimic the structure and function of actual organs. These are typically made by culturing (growing) stem cells on 3D extracellular matrices, or more recently via 3D bioprinting.
Limitations that currently hinder the large-scale adoption of organoids include the small obtainable tissue size (max. 5 mm), the dependence on animal-derived extracellular matrices, and the lack of a microenvironment to simulate in-human conditions accurately.
We see an opportunity for early-stage companies to drive the large-scale adoption of organoids through the development of novel culture systems with plant-based matrices to allow for the growth of larger organoids using materials free from animal products, and the implementation of automation and robotics to enable high-throughput screening and scalability, making it easier to cost-effectively produce large quantities of organoids.
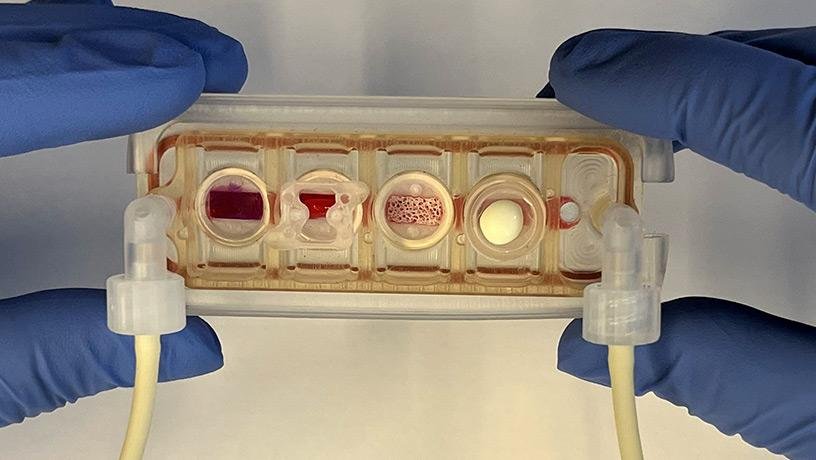
Organ-on-a-chip
Organ-on-a-chip devices are microscale platforms that replicate the functions of human organs on tiny USB-sized chips. These devices incorporate living cells and microfluidic systems to simulate the complex interactions that occur within organs.
While most ongoing research revolves around the optimisation of single-tissue or barrier tissue models, the forefront of organ-on-a-chip research allows for multi-organ models which can capture inter-tissue dynamics. For example, a group at Columbia University published their work in Nature Biochemical Engineering on a tissue-chip system in which four distinctly different human tissues (heart, liver, bone and skin) were linked by recirculating vascular flow. Each tissue was grown with its own optimised environment, while also receiving communication from signalling proteins and circulating cells.
Today, many well-established organoid and organ-on-a-chip companies are populating the alternative methodology space. In 2013 Leiden University spin-out MIMETAS was founded, offering multiple organ-on-a-chip models for high-throughput and AI-driven analysis. The company now works with nearly all major pharmaceutical companies and many top-tier academic institutes.
Emulate, a spin-out from the Wyss Institute for Biologically Inspired Engineering at Harvard University, has been leading the way in organ-on-a-chip developments. This Series E company has raised $225 million to date from investors such as Founders Fund, Northpond Ventures and Starlight Ventures.
Emulate completed the single largest organ-on-a-chip study to date demonstrating that the use of the human liver-chip could improve patient safety and better predict drug toxicity than other methods for modelling drug-induced liver injury. They have also developed a range of organ-on-a-chip models, including ones for the brain, colon, liver and lungs.
As many diseases can often affect multiple organs, the US-based startup Hesperos Inc. has developed ‘human-on-a-chip’ solutions using serum-free cell medium and gravity flow systems to create multiple interconnected organs. Thus far, they have designed systems that cover both the primary organs, such as the heart, liver and skeletal muscle, as well as barrier tissues, such as the skin, blood-brain barrier and gastrointestinal tract.
In a groundbreaking milestone last year, Hesperos assisted French pharmaceutical company Sanofi in receiving approval from the FDA for a clinical trial to extend the application of an existing drug to a rare autoimmune condition called Chronic Inflammatory Demyelinating Polyneuropathy, based on efficacy data derived solely from their organ-on-a-chip model. This marked the first drug to enter clinical trials based only on data derived from studies using organs-on-a-chip as a substitute for animals.
Hesperos and Sanofi’s success also highlighted how the development of therapeutics for rare human diseases may benefit from organs-on-a-chip, where animal models do not exist. There are 7,000 rare human diseases, and only 400 are actively being researched due to a lack of animal models.
Our focus at Creator Fund extends beyond ventures centred solely on a single in vitro model asset. We seek companies looking to expand on in vitro models across diverse indications, allowing for horizontal expansion and the potential to pivot into a range of clinical domains including oncology, neuroscience and cardiology. This approach mitigates risks associated with relying solely on a singular asset.
In silico models
With the excitement surrounding Artificial Intelligence (AI) and Machine Learning (ML), it is unsurprising that these tools are now harnessed for predicting drug toxicity and efficacy. AI-ML methodologies can be trained using existing toxicity and efficacy data on molecular structures of specific compounds to predict how analogous compounds would behave in humans.
Recent progress in the field includes an algorithm developed by researchers at the Johns Hopkins Bloomberg School of Public Health in 2018. The algorithm accurately predicted toxicity in 87% of cases, against an average of 81% with traditional testing from animal models.
This year, the Chan Zuckerberg Initiative announced that they are building one of the largest computing clusters in the world to create a “virtual cell”, which would allow for the prediction of cellular responses and behaviour to different conditions. Potentially, this could unlock a new avenue for toxicity and efficacy prediction.
The ideal in silico alternative, or digital twin, to animal testing, should be able to provide predictions on all, if not most, of the numerous types of toxicity (or endpoints) that are evaluated. Some endpoints have public and commercial in silico alternatives, such as the Skin Sensitisation Defined Approach ITSv1 tool developed by Lhasa Limited, while others are more complex and require more expansive datasets to build suitable in silico models. Examples of the latter include repeat-dose toxicity studies and reproductive toxicity.
The effectiveness of in silico models hinges on the quality and quantity of the underlying training datasets. When building in this space, it is extremely beneficial to have established collaborations with pharmaceutical companies or research institutes. These collaborations are important for commercial traction, validation testing and access to training data.
Anticipating the future
Investment in alternatives to animal testing is on the rise. In November 2023, US-based startup Vivodyne raised a $38mm seed round led by Khosla Ventures. Vivodyne is growing human organs in the lab while using advanced multimodal AI for predictive drug testing. Their approach is fully automated, provides comprehensive readouts for endpoint determination and generates proprietary data to refine the multimodal AI model.
We are particularly excited about integrating in vitro and in silico models. From utilising ML to accelerate the development of organoids and organ-on-a-chip models to the generation of in silico models trained on proprietary datasets built from validated in vitro models. We expect that a combination of different models will advance the reliability and accuracy of these alternative methodologies.
At Creator Fund we look more closely at founding teams with an unfair advantage. Specifically, we like to see a co-founder proficient in ML and a co-founder with an in vitro or clinical background and a strong understanding of toxicity and/or efficacy.
We are also looking forward to the improvements in patient outcomes from these alternatives as non-animal models can be used to increase genetic diversity and the incidence of rare adverse events. For example, a rare adverse effect that occurs in 1 out of 10,000 people is sometimes only seen in phase III clinical trials. Having genetic diversity information stored in a biobank, which can be integrated with the in vitro and in silico models could create a preclinical testing strategy that accurately represents the diversity of the entire population.
Using alternative methodologies will enable drug developers to detect adverse effects much earlier in the development process, thereby saving money, reducing timelines, minimising risk to patients, and potentially saving 5 million animals in the EU from undergoing drug testing.
The challenge with all these approaches is that we still require governments to catch up and remove the imperative to test all new drugs on two different mammals. The FDA’s approval last year and the EU’s plans to phase out animal testing indicate momentum for this regulatory change and we are excited to see the positive outcomes that will emerge from these shifts in approach.
If you are building new technologies for the study of drug toxicity and/or efficacy, we encourage you to reach out to the Creator Fund team. We are keen to learn more about what you are building!
Jun Jie (JJ) Ong is a PhD in Pharmaceutical Science at UCL, researching novel materials and predictive modelling for the 3D printing of personalised medicines and medical devices. He is a student investment partner and student lead of the Life Sciences team at Creator Fund.
Alice Pettitt is a PhD in Molecular Biophysics at UCL and King’s College London. Her research focuses on the characterisation of flexible viral proteins using a combination of nuclear magnetic resonance spectroscopy and molecular dynamics simulations. She is a member of the Creator Fund Life Science team.
With contributions from Meike Bleeksma
